Causes and Consequences of Snake Venom Variation – Casewell et al. 2020
Abstract:
1.8 million annual snake bite victims. This review describes how venom varies and its effects on snake bite victims.
Snake venom and Snakebite:
– Venom causes 138,000 deaths worldwide and an additional 500,000 additional cases of venom induced morbidity. It is listed as a Neglected tropical Disease by WHO. The biggest issue is that there is a level of variation both inter and intraspecifically thus needing a lot of different antivenoms for the same species.
Ecology Drives Inter and Intra specific snake venom variation:
Only members of these 3 clades can really do any harm to humans – 1. Elapidae (cobras, mambas, sea snakes, taipans) 2. viperidae (vipers and pit vipers, adders and rattlesnakes) 3. subfamily atractaspidinae (mole snakes/stiletto snakes). Certain families of snakes have structured their venom around specific toxin components thus vary in comparison and is even seen with the same species but different populations, usually when there is a diet change. For example even juveniles of the same specie may focus more on different prey and different prey handling techniques that requires potency in a different manner compared to adults who bite and release and then track. Venom also constantly changes as a result of neutral evolution (genetic drift), positive selection (Darwinian Selection) and ecological variance as a result of dietary preferences.
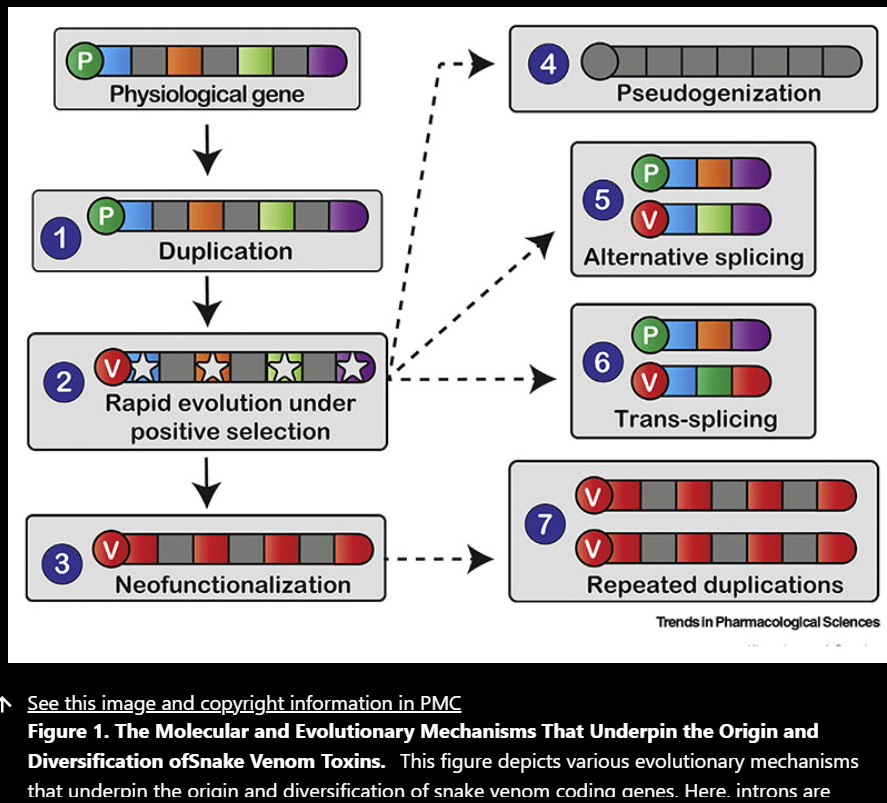
The Processes that Underpin Venom Variation
Numerous mechanisms have been proposed to explain the origin and diverse diversification of toxins which include: gene duplication, domain loss, evolutionary tinkering of expression levels, alternative and trans-splicing, and rapid evolution under positive Darwinian selection. Gene duplication mainly plays a role in phenotypic complexity and functional innovation which has been implicated in venom diversification. Repeated duplication events can cause some genes to undergo pseudogenization into dysfunctional forms and then deleted while others evolve new functions and are retained. Gene duplication can also alter the expression of genes other than just introducing novelty. Concerted evolution maintains high levels of sequence conservation in duplications even through recombination to increase expression of encoded types. This is not seen in snakes though. Domain loss is known to mediate toxin neofunctionalization.
Many of the toxin encoding genes are known to rapidly accumulate nonsynonymous substitutions and even though the protein structures of the venom components are conserved, the outer edges are in constant exposure to foreign material. Also purifying selection tries to ensure that the structure is conserved b/c who knows if the add-ons at the ends are going to make the protein not functional, but during environmental shifts for example from stress the purifying selection dwindles allowing for diversification.
Functional Consequences of Venom Variation:
Not every venomous snake has venom with all the components, rather each venom is a unique mixture of compounds. Most toxins that are found in venom include: 3 Finger Toxins, PLA2s, SVMPs and SVSPs. Each species of snake also does not hold all the venom components, it holds a mixture of specific venom components that best suit its environment. This results in intraspecific variation in venom and can mean different venom profiles for the same species with only tens of miles apart in territories.
Therapeutic Consequences
Antivenom is made by injecting an animal with venom from multiple different types of venomous snakes for a prolonged period of time and then harvesting the hyper immunized antibodies from the horse our cow. When being exposed to multiple different types of venoms with components having different molecular weights, means that the antivenom secured from hyperimmunization may not be extremely effective for all venoms that were used. For example some antibodies may be more pronounced than others which may make it ineffective against a specific type of snake even though that snake venom was used as part of the antivenom procurement procedure. There are also some reports of cross-neutralizing with anti venom but this is rare, in which an antivenom from one species works to neutralized another. One study found that the antivenom of indian saw scaled vipers were being used for west African saw scaled vipers and this increased fatality rates by 7x. It is hard to determine how effective the antivenom would be prior to use.
A big problem is that most of the antivenom facilities normally harvest snake venom from one single geographical region which is not good enough to get a diverse range of venom components.
Can Novel Snakebite Therapeutics Circumvent Venom Variation?
Next generation antivenom strategies include: monoclonal antibodies, nanobdies, small molecular inhibitors, aptamers and peptides, metal ion chelators, and synthetic immunogens. Some of the modalities listed previously are similar to old antivenom procurement procedure by using animal antibodies but some of them are completely artificial and synthetic. For example a mix of the two strategies was when a study injected a horse with a short neurotoxin chain that inhibited the toxicity in certain elapid snakes, and the antivenom acquired was actually working broadly against the designated species.